ABSTRACT
Contagious caprine pleuropneumonia (CCPP) is a fatal disease of goats occurring in many countries of Africa and Asia where the total goat population is more than 500 million. Vaccination is the most cost effective technique in the control of CCPP than any other control measures. In National Veterinary Institute (NVI) inactivated mycoplasma protein based vaccine obtained by centrifugation has been in use since many years. This study focuses on evaluating the safety and immunogenicity of inactivated whole culture CCPP vaccine currently developed in the NVI. Twenty six Mycoplasma capricolum subspecies capripneumoniae (Mccp) antibody free goats were used to evaluate the safety and immunogenicity of inactivated whole culture CCPP trial vaccine. The trial vaccine was prepared from culture of Mccp vaccinal seed grown in Mycoplasma specific hayflick media using spinner bottle. The protein content for one milliliter of whole culture was checked and found to be more than the minimum recommended dose (0.15 mg per dose). The culture was inactivated by 37% formalin at proportion of 0.5% of whole culture and adjuvanted by saponin at final concentration of 0.3%. The experimental animals were distributed into four groups: The group A consist of five goats for safety and all the other groups consists of seven animals each with group B for trial vaccine, group C for positive control and group D for negative control for immunogenicity trials. The goats were observed for two months for safety and immunogenicity evaluation during which serum samples were collected for immunogenicity and tested by using competitive Enzyme Linked immunosorbent Assay (cELISA) test. The results indicated that out of 7 goats vaccinated with trial vaccine, the mean sero-positivity was 60.71% while 7 goats vaccinated with the positive control showed mean sero-positivity of 58.86%. The analysis showed no significant difference between mean sero positivity of trial vaccine and positive control (P>0.05) as indicated by sero-conversion. The mean percent inhibition (PI) of trial inactivated whole culture CCPP vaccine vaccinated goats was 61.52% while the mean PI for positive control vaccine vaccinated group was 51.86%. In contrast the non-vaccinated controls showed mean PI of 40.65% which is significantly less than percent inhibition of the vaccinated groups (p=0.000). The body temperature and clinical observation of safety tested animals and other immunogenicity tested goats showed absence of any abnormality after vaccination both in vaccinated and controls. This study which was novel in its nature concluded that the trial inactivated whole culture ccpp vaccine is equally immunogenic as that vaccine already in use, the non-whole culture concentrated CCPP vaccine, and could be used for mass vaccination after conducting field immunogenicity trial.
Key words: Inactivated whole culture ccpp vaccine/goats/spinner bottle/safety/immunogenicity/NVI
Contagious caprine pleuropneumonia (CCPP) is a severe disease of goats occurring in many countries of Africa and Asia where the total goat population is more than 500 million (Acharya, 1992). A classical, acute CCPP is caused by Mycoplasma capricolum sub species .Capripneumoniae originally known as the F38 biotype (Mccp) (Leach et al., 1993; Mac Martin et al., 1980). Typical cases of CCPP are characterized by extreme fever (41 to 43°C), high morbidity and mortality rates in susceptible herds affecting all ages and both sexes. The clinical symptoms of the disease include abortion of pregnant goats, accelerated and painful respiration sometimes accompanied by a grunt, frequent, violent and productive coughing and in the terminal stages the goats are unable to move making them stand with their front legs wide apart, stiff and extended neck. The post-mortem examination of affected animals reveals fibrinious pleuropneumonia with massive lung hepatisation and pleurisy, accompanied by accumulation of straw-colored pleural fluid (OIE, 2014)
CCPP was first reported in Europe in 2004(Ozdemir et al., 2005) while the presence of CCPP in Ethiopia had been suspected since 1983 and confirmed later in1990 by isolation and identification of Mccp from the outbreak of CCPP in Ogaden, eastern Ethiopia (Thiaucourt et al., 1992). Since then the disease has been known to be endemic in different regions of the country (Sharew et al., 2005). Outbreaks of CCPP have been reported from almost all regions of the country especially from low land areas which are known as goat rearing regions (APHRD, 2010). Despite the presence and economic importance of CCPP in Ethiopia, the exact prevalence and distribution in the country has not been studied yet. However, few prevalence studies report showed CCPP prevalence as 31.6% by Lakewu et al. (2014) in Borana pasteural area, 4.92% by Yousuf et al. (2012), Dire Dawa, 32.36% by Sherif et al. (2012) in JigJiga. This indicates that the CCPP is one of the serious problem affecting goats population in the studied areas.
The disease can be controlled by the use of inactivated CCPP vaccine that has the ability to protect goats from infection for fourteen months (OIE, 2014; Litamoi et al., 1989). Despite the efficacy of this vaccine in Ethiopia, there are some constraints like the fastidious nature of the Mccp seed, less advanced technology used, the slow process of centrifugation or concentration employed to produce the existing CCPP vaccine makes Ethiopia not to satisfy its customers’ demand with regard to CCPP vaccine production. Most studies tried so far did not bring promising output to address this problem. However, some pilot study made at NVI indicated the possibility of getting adequate antigen per millimeter of whole culture of Mccp seed which urges us to launch this study to see the safety and immunogenicity of whole culture CCPP vaccine. Therefore, this study was designed with aim to evaluate the safety and immunogenicity of whole culture CCPP inactivated vaccine in comparison to the existing CCPP inactivated vaccine produced by concentration of cultural antigen.
Study area
This experimental study was conducted from September 2014 to April 2015 at National Veterinary Institute in Bishoftu town which is situated 47 km south east of the capital city, Addis Ababa. It was found at 9°N latitude and 4° E longitudes at an altitude of 1850 m above sea level in central highlands of Ethiopia (NMSA, 1999). National veterinary Institute is the sole veterinary vaccine producing laboratory in Ethiopia and currently it produces over 150 million doses of veterinary vaccines each year.
Antibody screening and husbandry of experimental animals
Twenty six male goats older than six months of approximately equal age with history of no previous exposure to CCPP and negative for Mccp specific antibodies were used for this experiment. Clinically healthy goats were purchased from local live- stock market and were kept in clean clear sheds. All the animals were screened for Mccp antibody by using c-ELISA. The animals declared negative for CCPP were treated by Albendazole and oxytetracycline and left for two weeks for adaptation. They were offered appropriate feeds like wheat bran, maize and Alfalfa, and clean water ad libitum.
Experimental design
Culture preparation
New stock of Mccp seed was obtained from vaccine seed bank (NVI) and passaged three times at weekly interval in hayflick media. The culture showing adequate change in PH (6.65-6.95) and free from contamination was allowed to pass to the next passage. Inoculum from the last passage was inoculated to production media in proportion of: 20% seed culture, 20% horse sera and 60% hayflick media. The mixture was incubated with continuous slow agitation in spinner bottle (90-100 rpm) at 37°C for 7 to 10 days until the desired turbidity and pH (6.4-6.8) was achieved. Sample was taken aseptically, smeared on slide, stained and observed for presence of any contaminant before proceeding to the next step (OIE, 2014).
Protein estimation for the whole culture
To estimate the protein content of the whole culture serious steps were carried out: the Mccp culture with adequate level of growth having optimum pH and purity was inactivated by 0.5% formalin and homogenized by shaking. Then, 100 ml Mccp culture was taken as sample and centrifuged at 20,000 rpm during which the supernatant was discarded and the pellet was washed three times to remove remnant of media. The amount of Mccp antigen was determined for this 100 ml sample and checked whether it had sufficient antigen (great or equal to 0.15 mg per ml of whole culture) by using bicinchoninic acid (BCA) assay. In this BCA assay 50 µl of known Bovine serum albumin (BSA) standards and 50 µl test samples (Mccp pellet suspension) and BCA and Copper sulphate were used and mixed as follows. Triplicate 50 µl of BSA and pellet suspension were each half fold diluted by using phosphate buffered saline (PBS), and 150 µl of bicinchoninic acid Cupper (II) sulphate (CuSO4) mix (in proportion of 50:1) was added to each well of U-shaped micro-plate. The plate was then incubated for 1 h at 37°C, and then OD of the reaction was read in ELISA reader at 562 nm wavelength. The regression line formula was drawn from average OD of the standard, and average protein concentration was calculated for the pellet suspension of Mccp inactivated whole culture from the regression line formula.
Adjuvant preparation
Saponin (Quillaja saponin, Guiness product) stock solution was prepared in 10% concentration with PBS and sterilized by steam autoclaving at 121°C for 15 min. The sterility of this stock saponin solution was checked by culturing it on .SBCDM agar and tryptose soya broth, thioglycollate broth and sabouraud agar media.
CCPP Inactivated whole culture trial vaccine formulation
The Mccp culture that had protein antigen content greater or equal to 0.15 mg/ml of whole culture was adjuvanted by adding saponin to make final concentration of saponin to 0.3% and then dispensed in 100 ml volume in polypropylene vials. The prepared vaccine was subjected to safety and other quality control tests (OIE, 2014).
CCPP inactivated whole culture trial vaccine quality control
Purity: The formulated vaccine was checked for presence of contaminants, by culturing a sample from the formulation on sterility test media including SBCDM agar and tryptose soya broth, thioglycollate broth and sabouraud agar media. The samples were also checked by Gram staining for bacterial contaminants.
Inactivation test: For inactivation test ten tubes marked from 1 to 10 each containing 10 ml of hayflick media were taken. A volume of 1 ml of inactivated whole culture was inoculated to four tubes while four tubes were inoculated with live Mccp inoculums as a positive control and the other two tubes were kept as negative control containing only media. All the tubes were incubated at 37°C for 10 days and observed for the presence or absence of mycoplasma growth.
Safety: Safety of the trial vaccine was checked by injecting three goats with 2 ml by subcutaneouse route and 2 goats were kept as control. The vaccinated goats were examined daily for presence of disease symptoms and injection site reaction and rectal temperature was recorded twice daily for 14 days at 9:am and 3:pm.
Experimental animals grouping and vaccination
After two weeks observation period, the experimental animals (n=26) which were Mccp antibody free were randomly allocated into four experimental groups as follows: Group A consists of five (n=5) goats which were used to evaluate the safety of the whole culture trial vaccine, group B consists of seven (n=7) goats which were vaccinated with trial whole culture inactivated CCPP vaccine and group C consists of seven (n=7) goats which were vaccinated with CCPP vaccine which was used as positive controls. On the other hand the remaining seven goats (n=7) where left as non-vaccinated controls in group D being injected with 1 ml sterile hayflick media (as placebo).
Follow up of vaccinated goats and blood sampling
Once the animals were vaccinated for immunogenicity test, the rectal temperatures of study goats were recorded for 7 days twice daily at 9:am and 3:pm. 5 ml blood was collected in vacutainer tube without EDTA from each experimental goats once per week at days 7, 14, 21, 28, 35, 42,49 and 56 of post vaccination. The blood samples were allowed to stand for 30 to 45 min and centrifuged at 3000 rpm for 3 min to extract clear serum (Tuck et al., 2009). The collected serum was stored at -20°C until processing for sero conversion by using c-ELISA test method (IDEXX, product) at Research and Development laboratory of NVI, Ethiopia.
Data analysis
The data regarding body temperature follow up and immunogenicity parameters were entered in Microsoft office Excel computer program and then summarized first by using descriptive statistics. Further statistical analysis was performed using statistical package for social science (SPSS)-version 20. The post-vaccination sero- conversion per group was calculated as the proportion of cELISA positive animals to the total test animals per group as percentage. Comparison of the rectal temperature data of safety tested goats and the mean percentage sero-conversion in different groups was done by using independent sample t-test. The mean percent inhibition of sero-conversion of immunogenicity trial groups, the temperature data of immunogenicity tested groups and the mean weekly percent inhibition of sero-conversion were compared by using analysis of variance (ANOVA). In all the analyses, confidence interval was at 95% and desired level of precision was 0.05.
Safety test
During these all observation periods, the animals showed no abnormal situation except minor swelling at the injection site which subsided within a week. The body temperature recorded for safety tested goats was analyzed and it showed that the body temperature remained within recommended range for healthy goats (37.5 to 40.5°C) and there was absence of significant difference between the vaccinated and non-vaccinated goats (P>0.05) (Tables 1 and 2). Besides these five animals, the three groups tested for immunogenicity were also checked for their body temperature difference for the first 7 days after vaccination. The temperature of both control and inoculated animals was almost same and no significant difference (P>0.05) was found as shown in Tables 3 and 4.
Immunogenicity test
For the period of eight weeks sero-conversion, ability of goats categorized under the three treatment groups were analyzed. Accordingly, the comparison of mean percent positive of sero-conversion for eight weeks duration of trial vaccine and positive control and also comparison of mean percent inhibition for eight weeks in the three groups were given in Tables 5 and 6.
As the above PI comparison showed presence of significant difference (P< 0.05) among the three treatment groups, to know between which groups significant difference exist, multiple comparison was made (in post hoc analysis of ANOVA) and the result is given as follows (Table 7).It showed that mean PI of whole culture is the highest (61.52±2.92) and the mean PI of positive control (51.86±4.95) is greater than that of negative control (40.65±2.84).
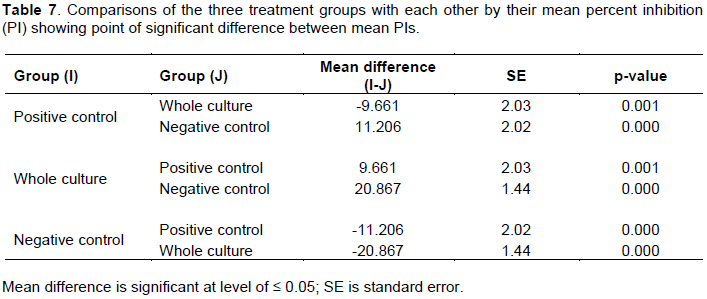
The above pair wise comparison by ANOVA indicated that the presence of significant difference among the mean PI value in the test groups. This means, there was strong significant difference between whole culture vaccinated and positive control vaccinated where the PI of whole culture vaccinated was greater than the PI of positive control vaccinated and the PI of non-vaccinated controls was significantly less than both vaccinated groups. After this observation that means PI was significantly different among the three treatment groups (Tables 6 and 7). Table 8 shows weekly summary statistics for PIs for three treatment groups for 8 weeks observation periods.
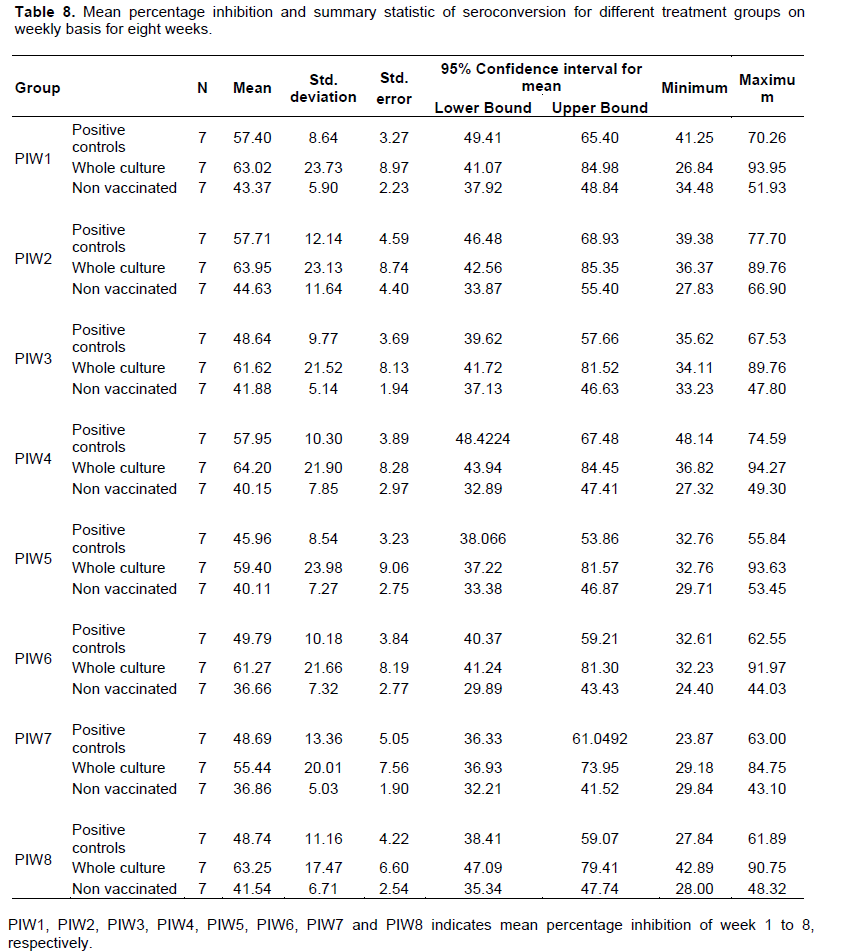
When the above mean weekly observation is depicted graphically the mean percentage inhibition of the whole culture was above both the mean percentage inhibition of positive and negative controls in all weeks. In some cases, the mean percentage inhibition of positive control failed below cut off value of c-ELISA (<50) but the mean Percentage inhibition of the negative control was as expected (below 50 throughout the weeks when the data was collected) (Figure 1).
In Ethiopia goat rearing has got important means of income especially in pastoral areas where goats are reared in large numbers (Hirpa and Abebe, 2008). Despite their importance goats have been known to be affected by tremendous infectious diseases which cost their life and contribution. CCPP is one of the most important infectious disease affecting goats contributing for high mortality and economic loss.
The main objective of this experimental study was to evaluate the safety and immunogenicity of inactivated whole culture CCPP trial vaccine in comparison to the inactivated CCPP vaccine which has been produced through other technique in NVI. Accordingly, trial inactivated whole culture CCPP vaccine was prepared based on standard operational procedure of CCPP vaccine production in NVI (Tesgera and Tefera, 2012; OIE, 2014) and the trial vaccine was subjected to all the quality control checks which were made according to OIE (2014). The safety test of this trial vaccine was made on goats prior to the commencement of the immunogenicity test. Analysis of rectal temperature for safety tests for 14 days twice daily showed no significant difference between the vaccinated and the control groups (p > 0.05) and these were within the normal goat body temperature (Radosits et al., 2007). This was also of the case for body temperature of immunogenicity tested animals where there was no significant difference between body temperature of whole culture vaccinated, positive control vaccinated and non-vaccinated controls (P > 0.05). It has been recommended that for the vaccine to pass safety tests, animals must not show significant abnormalities except minor swelling at the injection site which should subside within a week of observation (OIE, 2014). Hence, the current experiment proved the trial vaccine to be safe. The mean weekly sero-conversion (Mccp antibody positivity) of goats in three groups indicated that the whole culture vaccinated group had 60.71% (n=7) goats sero-positive, positive control vaccinated group had 58.86% (n=7) goats positive while the non-vaccinated control group showed no sero-positivity throughout two months of observation The sero-positivity between whole culture vaccinated group and positive control vaccinated group showed no significant difference (P > 0.05). This result indicated that the new trial vaccine has comparable merits to the existing NVI inactivated CCPP vaccine prepared by concentration method. Lakew et al. (2014) reported rise in these ropositivity after vaccination with inactivated CCPP vaccine at field level. However, it was impossible to compare the findings of the current study with study made elsewhere, as there was no previous works made on immunogenicity of inactivated whole culture CCPP vaccine and this is the first finding of its kind.
The mean percentage inhibition (PI) of sera of the three groups was also compared to evaluate the strength of sero-conversion during the experimental period. The result indicated that mean PI of whole culture was the highest (61.52±2.92), and the mean PI of positive control (51.86±4.95) was greater than that of negative control (40.65±2.84) and the difference was statistically significant (P=0.000). The reason needs further investigation but it might be attributed to quantity and type of antigen found in the inactivated whole culture CCPP trial vaccine which is whole in its nature and nothing was subtracted by concentration procedure of the existing vaccine (Tesgera and Tefera, 2012).
In this trial, immunized animals were able to produce antibody during the first week of vaccination and continued producing antibodies variably during the trial which lasted eight weeks. It has been known that the CCPP vaccine protect goats for one year (OIE, 2014).In conclusion the newly designed inactivated whole culture CCPP trial vaccine is equally safe and immunogenic as the existing inactivated CCPP vaccine produced by concentration technique. But as the production procedure of the trial vaccine is easier, requires less time and is not capital investment intensive, the institute can shift its production strategy to the new methodology even though the correlation between this sero-conversion and field protection study remained to be done. Additionally the strength of antibody titer (as was seen by mean percentage inhibition) of whole culture trial vaccine was higher than that the vaccine used as positive control based on serological test(c-ELISA). So this inactivated trial whole culture CCPP vaccine could provide greater protection of goats than those vaccinated by existing vaccine but this needs further investigation by challenge study in the future.
The authors have not declared any conflict of interests.
REFERENCES
|
Acharya RM (1992). Recent advances in goat production. Fifth International Conference on Goats, New Delhi, India, Pp.49-94.
|
|
|
|
APHRD, Ethiopia Animal Health yearbook (2009). Animal and Plant Regulatory Directorate, Ministry of Agriculture, Addis Ababa, Ethiopia.
|
|
|
|
|
Hirpa A, Abebe G (2008). Economic significance of sheep and goats. In: sheep and goats production handbook for Ethiopia. Ethiopian sheep and goats productivity improvement program. pp. 1-4.
|
|
|
|
|
Lakewu M, Sisay T, Ayelet G, Eshetu I, Dawit G, Tolossa T (2014). Seroprevalence of contagious caprine pleuropneumonia and field performance of inactivated vaccine in Borana pasteural area, Southern Ethiopia. Afr. J. Microb. Res. 8(24):2344-2351.
Crossref
|
|
|
|
|
Leach RH, Ern H, MacowanKJ (1993). Proposal for designation of F38-type caprine mycoplasmas as Mycoplasma capricolum subsp. capripneumoniae subsp.nov.and consequent obligatory relegation of strains currently classified as M. capricolum (Tully, Barile, Edward, Theodore, and Erno 1974) to an additional new subspecies, M. capricolum subsp. capricolum subsp. Nov. Int. J. Syst. Bacteriol. 43:603-605.
Crossref
|
|
|
|
|
Litamoi JK, Lijodi FK, Nandokha E (1989). Contagious caprine pleuropneumonia: some observations in a field vaccination trial using inactivated Mycoplasma strain F38. Trop. Anim. Health Prod. 21:146-150.
Crossref
|
|
|
|
|
Mac Martin, DA, Macowan, KJ. Swift, LL (1980). A century of classical contagious caprine pleuropneumonia: from original description to etiology. Br. Vet. J. 136:507-515.
|
|
|
|
|
NMSA (1999). Rainfall and temperature data of Debre Zeit. National Meteorological Service Agency, Addis Ababa, Ethiopia pp. 1-17.
|
|
|
|
|
OIE, Manual: World organization for Animal Health (2014). Contagious caprine pluropneumonia. In Manual of Diagnostic tests and vaccines for terrestrial animals.,chapter pp. 270-282.
|
|
|
|
|
Ozdemir U, Ozdemir S, march J, Churchwood C, Nicholas RAJ (2005).Outbreaks of CCPP in the Thrace region of Turkey. Vet. Rec. 156:286-287.
Crossref
|
|
|
|
|
Radostits OM, Gray CC, Hinchliff KW, Constable PD (2007). A textbook of the disease of cattle, sheep, pig and goats.10th edition, Saunders LTD. Vet. Med. 10:2045-2050.
|
|
|
|
|
Sharew AD, Staak C, Thiaucourt F, Roger F (2005). Serological investigations into contagious caprine pleuropneumonia in Ethiopia Trop. Anim. Health Prod. 37:11-19.
Crossref
|
|
|
|
|
Sherif M, Addis M, Tefera M (2012). Contagious caprine pleuropneumonia: Serological survey in selected district of Jijjiga Zone, Ethiopia. Asian J. Anim. Sci. 6(6):309-315.
Crossref
|
|
|
|
|
Tesgera T, Tefera B (2012). Standard operating procedure for production of contagious caprine pleuropneumonia vaccine in NVI
|
|
|
|
|
Thiaucourt F, Breard A, Lefevre P.C, Mebratu GY (1992). Contagious caprine pleuropneumonia in Ethiopia. Vet. Rec. pp. 131-585.
|
|
|
|
|
Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, Wang W, Brenner DE (2009). Standard operating procedures for serum and plasma collection: Early detection Research network consensus statementstandard operating procedure integration workshop group. J. proteome Res. 8(1):113-117.
Crossref
|
|
|
|
|
Yousuf E, Melaku A, Bogale B (2012). Seroprevalence of Contagious Caprine Pleuropneumonia in DireDawa Provisional Administrative council, Eastern Ethiopia. J. Vet. Med. Anim. Health 4(7):93-96.
|
|